
Some examples are:Ĭommon names such as these often have their origin in the history of the science and the natural sources of specific compounds. The relationship of these names to each other is usually arbitrary, and no rational or systematic principles underly their assignments. These names have remained in common use, and are widely recognized. Just as each distinct compound has a unique molecular structure which can be designated by a structural formula, each compound must be given a characteristic and unique name.Īs organic chemistry grew and developed from its early beginnings, many compounds were given trivial names at the time of their discovery. The increasingly large number of organic compounds identified with each passing day, together with the fact that many of these compounds are isomers of other compounds, requires that a systematic nomenclature system be developed. Simply put, aliphatic compounds are compounds that do not incorporate any unsaturated aromatic rings in their molecular structure. They are also members of a larger class of compounds referred to as aliphatic. Alkanes and cycloalkanes are termed saturated, because they incorporate the maximum number of hydrogens possible without breaking any carbon-carbon bonds. Although these hydrocarbons have no functional groups, they constitute the framework on which functional groups are located in other classes of compounds, and provide an ideal starting point for studying and naming organic compounds. Hydrocarbons of this kind are classified as alkanes or cycloalkanes, depending on whether the carbon atoms of the molecule are arranged only in chains or also in rings. From the previous discussion of formula analysis, the formulas for such hydrocarbons will be C nH (2n+2–2r), where n is the number of carbon atoms and r is the number of rings.
Alkane functional group full#
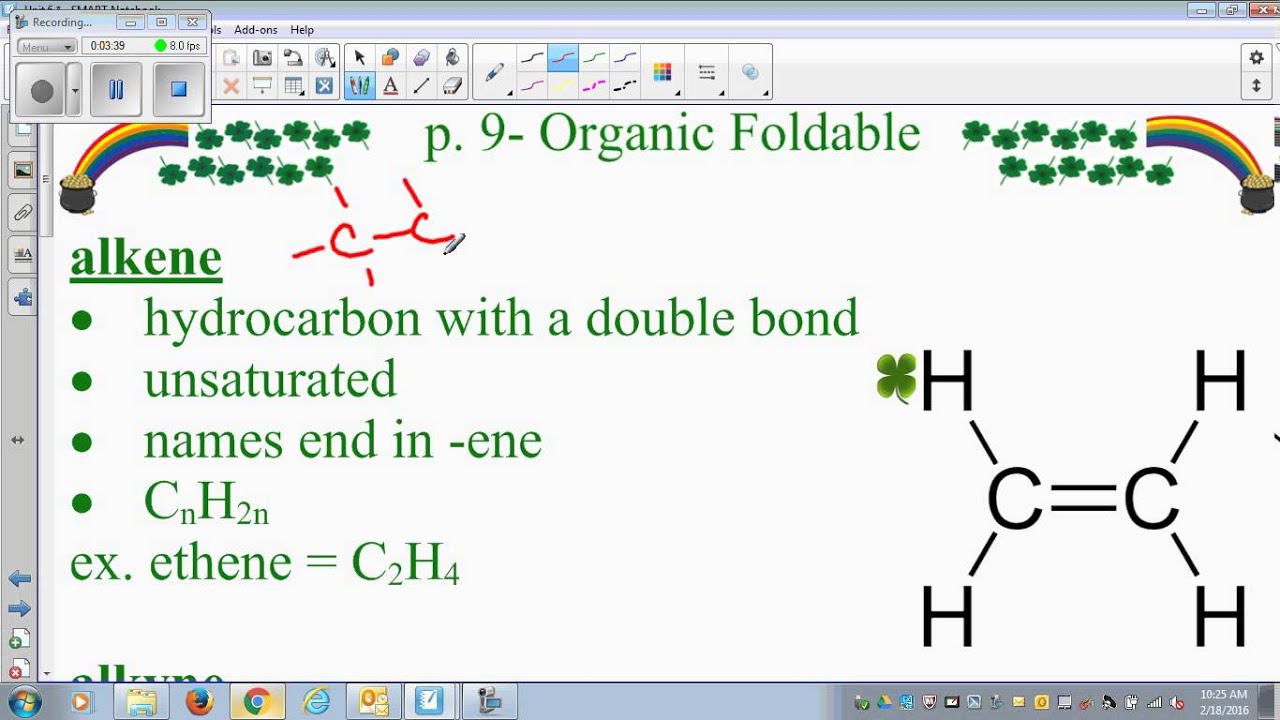
Such compounds are necessarily hydrocarbons, made up of chains and rings of carbon atoms bonded to a full complement of hydrogen atoms (all carbons are sp 3 hybridized). In order to establish a baseline of behavior against which these reactions may be ranked, we need to investigate the reactivity of compounds lacking any functional groups. Most reactions of organic compounds take place at or adjacent to a functional group. Below is an image of multiple functional groups found in organic chemistry.Alkanes & Cycloalkanes Saturated Hydrocarbons Alkanes and Cycloalkanes In the formulas, the symbols R and R' usually denotes an attached hydrogen, or a hydrocarbon side chain of any length, but may sometimes refer to any group of atoms.
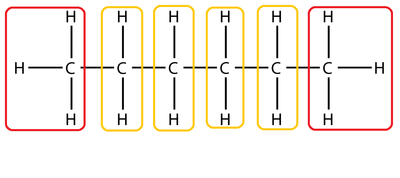
The following is a list of common functional groups.

Functional groups are far less stable than the carbon backbone and are likely to participate in chemical reactions. They determine the characteristics and chemical reactivity of molecules. The first carbon atom after the carbon that attaches to the functional group is called the alpha carbon.įunctional groups are attached to the carbon backbone of organic molecules.
Alkane functional group free#
When the group of atoms is associated with the rest of the molecule primarily by ionic forces, the group is referred to more properly as a polyatomic ion or complex ion-all of these are called radicals, by a meaning of the term radical that predates the free radical. The non- hydrogen atoms of functional groups are always associated with each other and with the rest of the molecule by covalent bonds.


 0 kommentar(er)
0 kommentar(er)
